Tuberculosis
I. Mycobacterium tuberculosis Pathogenesis
Tuberculosis, caused by Mycobacterium tuberculosis (Mtb), infects one-third of the world’s population and kills more than 1.5 million people every year globally. The main reasons for the failure to curb Mtb infection are the emergence of multidrug-resistant strains, lack of potent multi-target drugs, inadequate diagnosis, and the ability of the pathogen to evade host immune responses.
Our laboratory studies the molecular, genetic, and immunological mechanisms of mycobacterial pathogenesis and its interaction with lung macrophages and bone marrow stem cells. Several studies have shown that Mtb can also reside in bone marrow stem cells and use them as a safe niche to evade host immune responses. We also investigate the implications of cross-talk between stem cells and macrophages on the fate of Mtb infection.
Our research is focused on the following areas:
-
Modulation of macrophage and bone marrow stem cell immune responses during Mycobacterium tuberculosis infection:
Mtb is known to modulate host immune responses to promote its survival in macrophages and stem cells. Through molecular and cellular analyses, we study modulation of autophagy, antimicrobial peptide biosynthesis, and oxidative stress responses during Mtb infection. Peroxisomes play an important role in scavenging oxidative stress responses and modulation of the β-oxidation pathway. We are also interested in investigating molecular mechanisms regulating peroxisome biogenesis during Mtb infection in stem cells and macrophages.
-
Epigenomics of stem cells and macrophages:
One of the prominent mechanisms employed by pathogenic bacteria to facilitate their survival in host cells involves induction of various epigenetic modifications in host DNA, histones, and RNA. Mtb induces several transcriptional activation and repression epigenetic changes to promote its replication, propagation, and protection from host immune responses.
We investigate epigenomics, molecular mechanisms of epigenetic modifications, and their effects on immune functions of macrophages and stem cells during Mtb infection. Mesenchymal stem cells (MSCs) interact with macrophages to influence immune responses during Mtb infection. We also study how MSC–macrophage cross-talk shapes host defense mechanisms by promoting macrophage polarization (M2), ROS levels, and cytokine responses, thereby creating an immunosuppressive environment to support Mtb persistence.
-
Role of Calcium Ion Channels during M. tuberculosis infection:
Mtb alters calcium (Ca2+) channel function in immune cells. Ca2+ channels regulate processes such as lysosomal trafficking and autophagy, which are crucial for pathogen clearance. We have shown that M. bovis BCG increases, whereas virulent Mtb down-regulates Ca2+ channel expression in macrophages and neutrophils.
Blocking these channels showed better control of Mtb infection in Trpv4-/- knockout mice during the late infection phase. Our lab investigates the role of calcium ion channels in lung and bone marrow stem cell inflammatory responses. Our studies suggest that targeting these ion channels may offer a novel host-directed strategy to improve TB therapy and limit tissue damage.
-
Development of novel anti-M. tuberculosis therapeutic molecules:
Over the decades, several anti-TB drugs have been developed; however, due to the emergence of multidrug-resistant (MDR), extremely drug-resistant (XDR), and totally drug-resistant (TDR) Mtb strains, most of these drugs have become ineffective. Moreover, Mtb is an intracellular pathogen with a lipid-rich cell wall, limiting drug diffusion and effective killing.
To overcome these challenges, our group, in collaboration with other research groups, is working on the synthesis of novel biomolecules and drugs, studying their modes of action, and developing targeted delivery strategies to infected cells to improve treatment of drug-resistant tuberculosis.
-
Development of non-invasive tuberculosis diagnostics:
Mtb diagnostics are vital for early detection and control of tuberculosis. Current TB diagnostics are often time-consuming, less sensitive, less specific, and require sophisticated equipment and skilled manpower.
To address these limitations, we are developing non-invasive (saliva, sputum, and tongue swab), rapid, specific, and affordable diagnostic kits for the detection of active and drug-resistant tuberculosis, suitable for low-resource settings in endemic regions.
Leukemia Acute Lymphocytic Leukemia
II. Development of Novel Asparaginase Drugs to Improve Primary and Relapse Leukemia Therapy
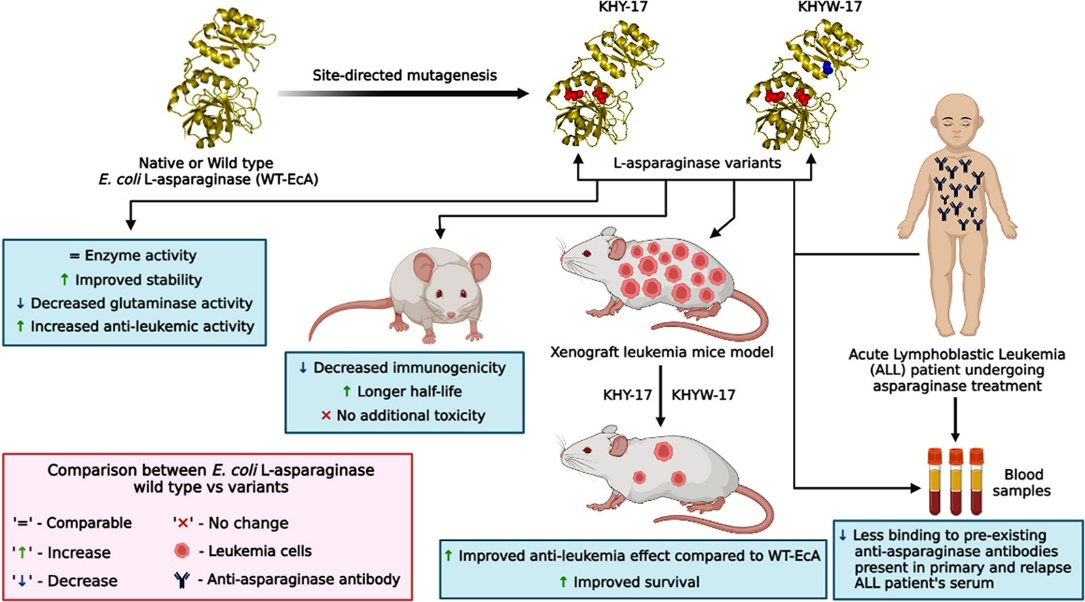
Asparaginases are central to the treatment of acute lymphatic leukemia (ALL), a disease affecting lymphocytes and their precursor cells in the bone marrow. However, current asparaginase drugs exhibit severe side effects, including immunogenicity, neurotoxicity, low stability, and reduced therapeutic efficacy in both primary and relapsed ALL.
Using a protein engineering approach, we have developed novel asparaginase molecules with improved therapeutic efficacy. Preclinical and pharmacological studies in ALL patient samples and mouse models showed that these novel molecules exhibit low immunogenicity and neurotoxicity, along with high stability. Importantly, they do not bind to pre-existing antibodies in ALL patients, suggesting potential use in relapsed ALL therapy.
These novel asparaginase molecules demonstrated improved in vivo pharmacokinetic properties compared to wild-type and commercial asparaginases. All molecules showed significantly enhanced therapeutic efficacy in NOD/SCID xenograft mouse models without causing acute or chronic toxicity.
In collaboration with D.K. Biopharma Pvt. Ltd., Mumbai, we have initiated scale-up production and Phase I/II clinical trials in ALL patients at a GMP facility. Currently, we are developing next-generation chemically modified L-asparaginase molecules to further improve leukemia therapy.


